Abstract
Purpose: To translate a recombinant peptide containing the amino-terminal fragment (ATF)of urokinase plasminogen activator receptor-targeted magnetic iron oxide (IO) nanoparticles(uPAR-targeted human ATF-IONPs) into clinical applications, we conducted a pilot study to evaluate the toxicity and pharmacokinetics of this nanoparticle in normal rhesus monkeys.
Keywords: uPAR-IONP, nonhuman primates, Transient harm, Self-healing
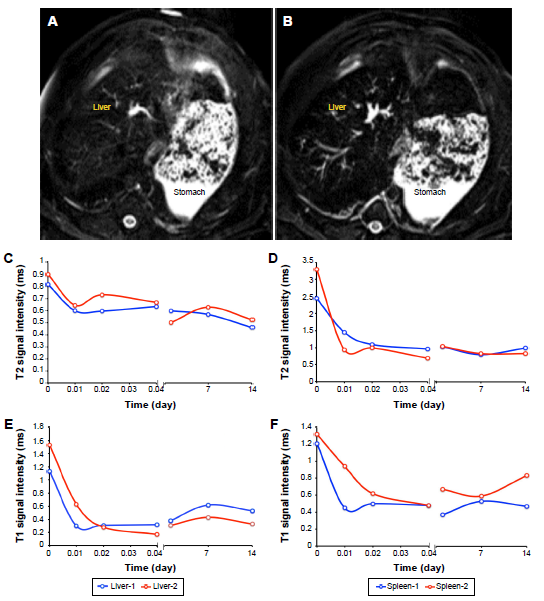
Fig.1. The MRI signal alterations in liver and spleen.
Notes: (A and B) are the MRI images before and after ATF-IONP injection, respectively, without and with PEG coating, both of them are phased from up to down. (C and D) are the variations of T2 signal intensity; (E and F) are the variations of T1 signal intensity. Monkey 1 received ATF-IONP, and Monkey 2 received ATF-PEG-IONP.Abbreviations: ATF, amino-terminal fragment; IONP, magnetic iron oxide nanoparticle; MRI, magnetic resonance imaging; PEG, polyethylene glycol.
Tel : 028-8592-1823 (in China)
Tel:+1-517-388-6508 (in US)
Tel : +86 (28) 8592-1823 (outside of China)
Fax : +86-28-62491302
Zip code : 610041
Address: No.88, Keyuan South Road, Hi-tech Zone, Chengdu, Sichuan Province, China